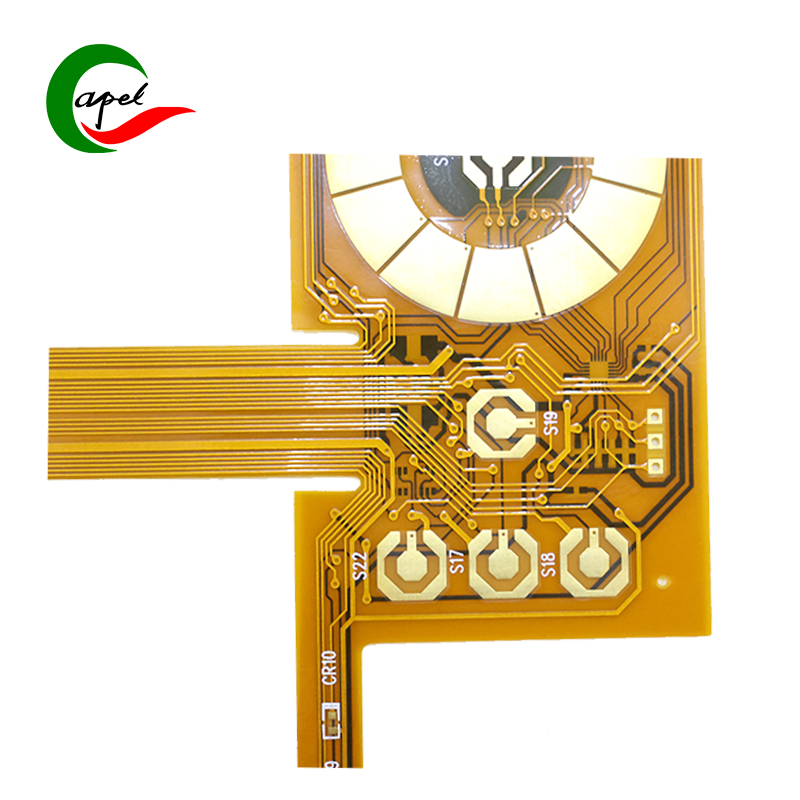
Kaboer’s Double-Sided Flexible PCB for Medical Devices is tailor-made for signal transmission and control units of core medical equipment such as large-scale medical imaging devices, critical care monitoring systems, surgical navigation equipment, and medical biochemical analyzers. It precisely meets the core demands of large-scale medical scenarios—high integration adaptation, long-distance stable transmission, sterilization resistance, and regulatory compliance. With a robust configuration of "medical-grade immersion gold finish + double-sided high-density wiring", it stands as a trusted choice for the R&D of medium-to-large medical equipment, empowering enterprises to build high-precision, high-stability, and fully compliant high-end medical solutions!
Featuring a double-sided flexible core structure, it perfectly balances the spatial layout and operational reliability of large medical devices: with an overall thickness of 0.23mm, the flexible substrate offers excellent bending resilience and fatigue resistance, flexibly fitting complex internal cavities, long-distance wiring paths, and module connection gaps of large equipment. It easily avoids wiring interference, adapting to the modular and integrated design needs of devices, and completely solving the pain points of traditional rigid PCBs (cumbersome wiring and poor adaptability) in large medical equipment; with precise dimensions of 114.11*236.16mm, the optimized large layout perfectly matches the multi-component integration needs of medium-to-large medical modules, providing ample space for interconnection of sensor arrays, drive units, display terminals, and other components; the 0.17/0.2mm fine line width/spacing and 0.2mm micro drill size enable high-density integration of signal and power channels, stably carrying long-distance transmission of multiple groups (e.g., physiological signals, control commands, imaging data) on the large-size substrate, and providing solid circuit support for high-precision operation of medical equipment.
Key advantages directly address pain points of large-scale medical scenarios, demonstrating medical-grade robustness: ① Equipped with medical-grade immersion gold finish, it offers superior oxidation resistance, resistance to sterilization wipes (compatible with medical disinfectants like alcohol, hydrogen peroxide, and povidone-iodine), and low contact resistance. It ensures high-speed, distortion-free long-distance signal transmission, eliminating diagnostic errors caused by signal attenuation or oxidation. Meanwhile, the immersion gold surface enhances soldering reliability, adapting to the high-cleanliness assembly standards of medical equipment; ② Double-sided wiring design significantly improves space utilization and transmission efficiency, with rational division of signal and power channels to strongly suppress electromagnetic interference (EMI). It avoids signal crosstalk among multiple modules inside large medical devices, guaranteeing the integrity and accuracy of imaging data and physiological parameter transmission, and complying with the IEC 60601 EMC standard for medical devices; ③ The flexible substrate strictly adheres to ISO 10993 biocompatibility requirements, boasting excellent wide-temperature resistance (-40℃ to 85℃), strong vibration resistance, and aging resistance. It can withstand long-term continuous operation, frequent sterilization cycles, and transportation jolts of large medical equipment, reducing downtime and maintenance risks, and aligning with the core demand of "low maintenance and high stability" in medical scenarios.
Backed by Kaboer’s years of medical-grade PCB R&D experience and strict quality control system, this double-sided flexible PCB is compatible with global medical regulatory requirements (FDA, CE, NMPA). It can be customized for circuit layout, interface form, and mounting holes based on the functional needs of large medical devices (e.g., signal transmission distance, interface type, number of integrated modules). Paired with rapid prototyping and efficient SMT assembly services, it helps customers shorten R&D cycles and accelerate product certification and market launch. Whether for high-definition data transmission of large imaging devices, multi-parameter real-time monitoring of critical care systems, or precise command transmission of surgical navigation equipment, it serves as the "core signal hub" of medical devices with core advantages of "regulatory compliance, stable signal transmission, and high integration adaptation", helping enterprises reduce compliance risks and enhance product credibility and market competitiveness!