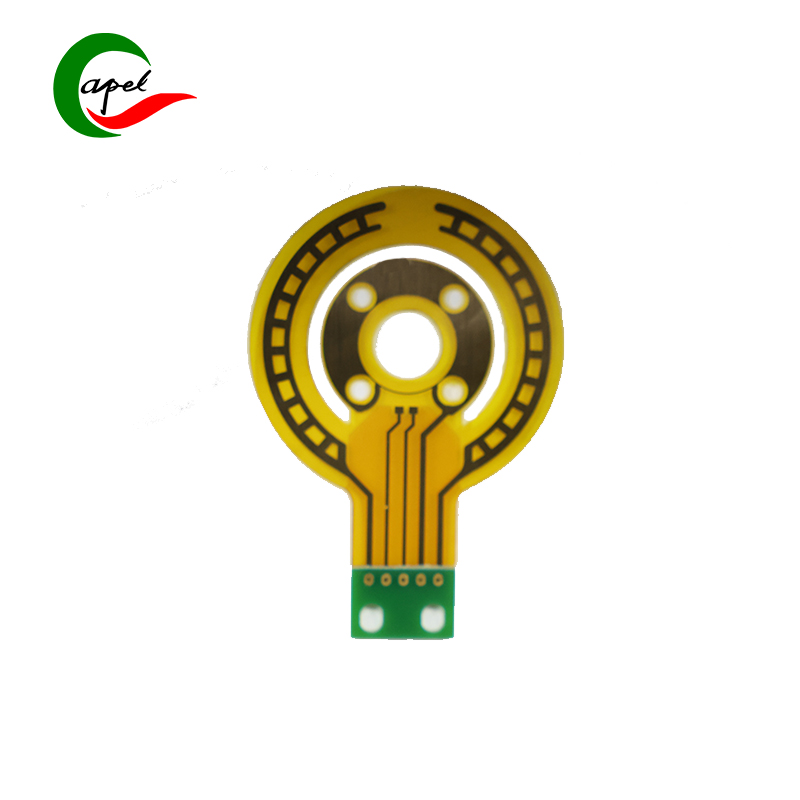
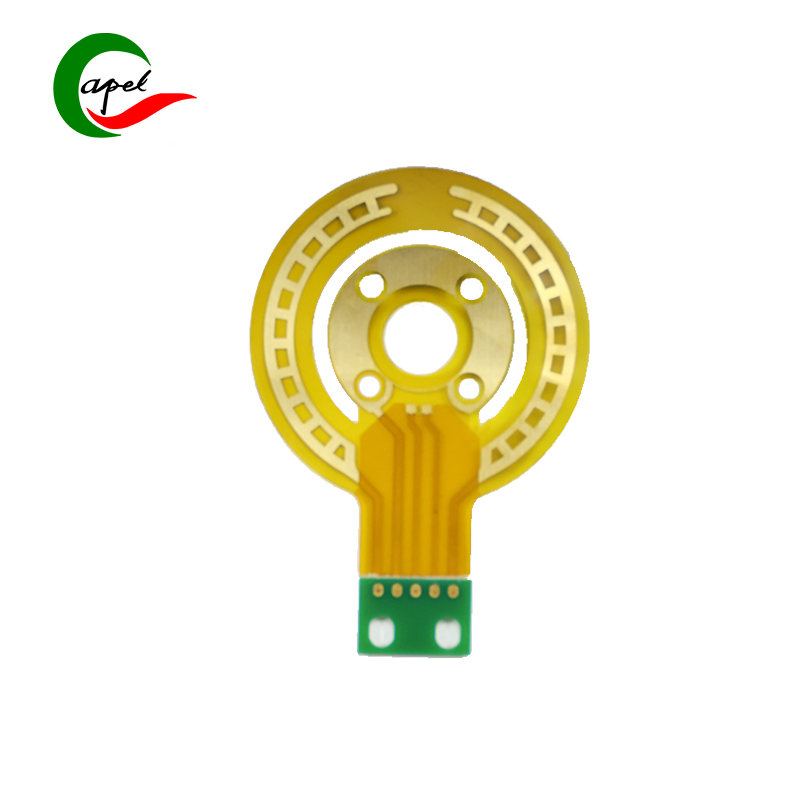
Kaboer’s 3-Layer Rigid-Flex Board for Medical Devices is tailor-made for core medical components such as bedside monitors, portable diagnostic equipment, minimally invasive therapy device control modules, and ultrasound probe assemblies. It precisely meets the core demands of medical scenarios—high reliability, sterilization compatibility, accurate signaling, and complex structure adaptation—setting the standard for medical-grade performance with rigid-flex synergy. As a trusted choice for medical device R&D, it empowers enterprises to deliver safe, precise, and compliant high-end healthcare solutions!
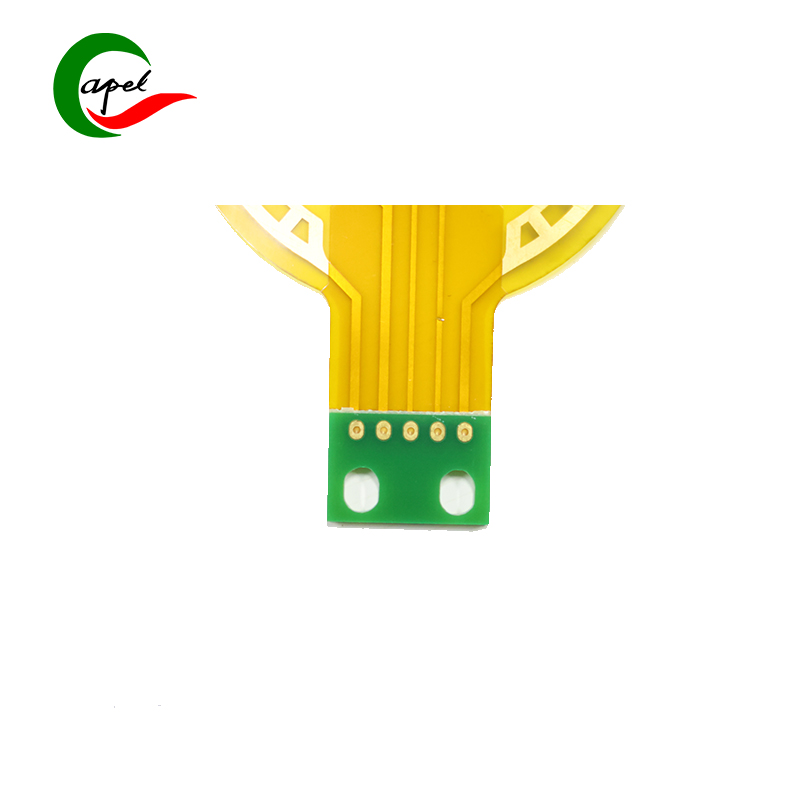
Adopting a 3-layer rigid-flex synergy structure, it perfectly balances adaptability and safety in medical equipment: the 0.17mm ultra-thin flexible layer offers exceptional bending toughness and fatigue resistance, flexibly fitting narrow cavities, curved structures, and moving joints (e.g., curved execution ends of minimally invasive instruments) inside medical devices. It easily avoids wiring interference, adapting to the compact layout of portable equipment and dynamic operation needs of minimally invasive instruments; the 1.7mm sturdy rigid layer meets the load-bearing and impact-resistant standards of medical devices, reliably supporting core components such as high-precision sensors, signal processing chips, and low-noise amplifiers. This achieves efficient integration of "flexible adaptation to complex device structures + rigid fixation of precision parts". With precise dimensions of 72.97*50.94mm, the optimized layout satisfies multi-component integration needs without redundant bulk, providing ample room for the miniaturization and lightweight design of medical devices.
Key advantages directly address medical scenario pain points, demonstrating medical-grade robustness: ① Full-board immersion gold finish delivers superior resistance to sterilization wipes (compatible with medical disinfectants like alcohol, povidone-iodine, and hydrogen peroxide), oxidation, and corrosion. Low contact resistance ensures high-speed, distortion-free transmission of physiological signals (e.g., ECG, SpO2, ultrasound images), meeting the long-term high-frequency use and sterile maintenance requirements of medical devices, and eliminating diagnostic errors caused by signal distortion; ② White characters solder mask + through-hole design features clear markings, facilitating sterile module assembly, rapid debugging, and post-maintenance in high-cleanliness production environments. It adapts to GMP production standards of the medical manufacturing industry, improving mass production efficiency and operational safety; ③ 3-layer scientific wiring layout rationally separates signal, power, and ground layers, building a strong anti-interference system. It effectively suppresses electromagnetic interference (EMI) and signal crosstalk, ensuring seamless collaboration across the full process of "data collection - signal processing - command execution" even in clinical environments with multiple coexisting devices. This guarantees the reliability of diagnostic data and therapeutic instructions, complying with the IEC 60601 functional safety standard for medical devices.
Backed by Kaboer’s years of medical-grade PCB R&D experience and strict quality control system, this 3-layer rigid-flex board strictly complies with ISO 10993 biocompatibility standards and is fully compatible with global medical regulatory requirements (FDA, CE, NMPA). It can be customized for interface types, protection levels, and wiring routes (e.g., low-noise signal path design) based on specific medical device needs. Paired with rapid prototyping (sample delivery within 7 days) and efficient SMT assembly services, it helps customers shorten R&D cycles and accelerate product launch and certification processes. Whether for precise signal collection of portable diagnostic equipment, long-term stable operation of bedside monitors, or safe control of minimally invasive therapy devices, it serves as the "core neural hub" of medical equipment with stable performance—helping enterprises reduce compliance risks and enhance product credibility and market competitiveness!