Kaboer’s 3-Layer Flexible PCB for Medical Devices is tailor-made for core medical components such as portable diagnostic instruments, minimally invasive therapy device control modules, monitor signal transmission units, and medical sensor interfaces. It precisely meets the core demands of medical scenarios—regulatory compliance, accurate signal transmission, sterilization resistance, and compact flexible adaptation. With a robust configuration of "medical-grade tin plating + 3-layer high-density wiring", it stands as a trusted choice for medical device brands to develop highly reliable products, empowering improved precision and safety in diagnosis and treatment!
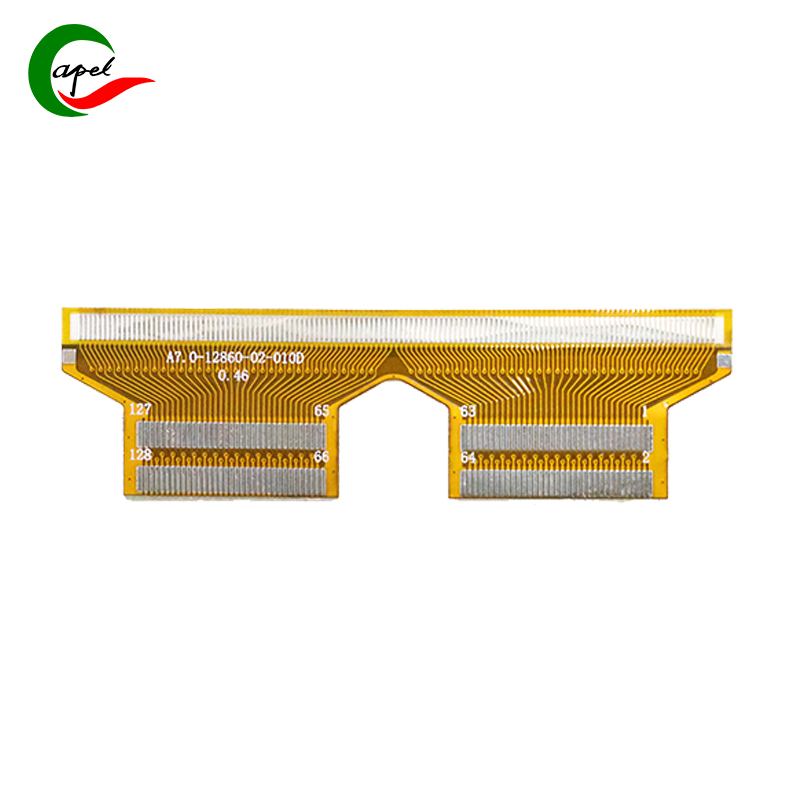
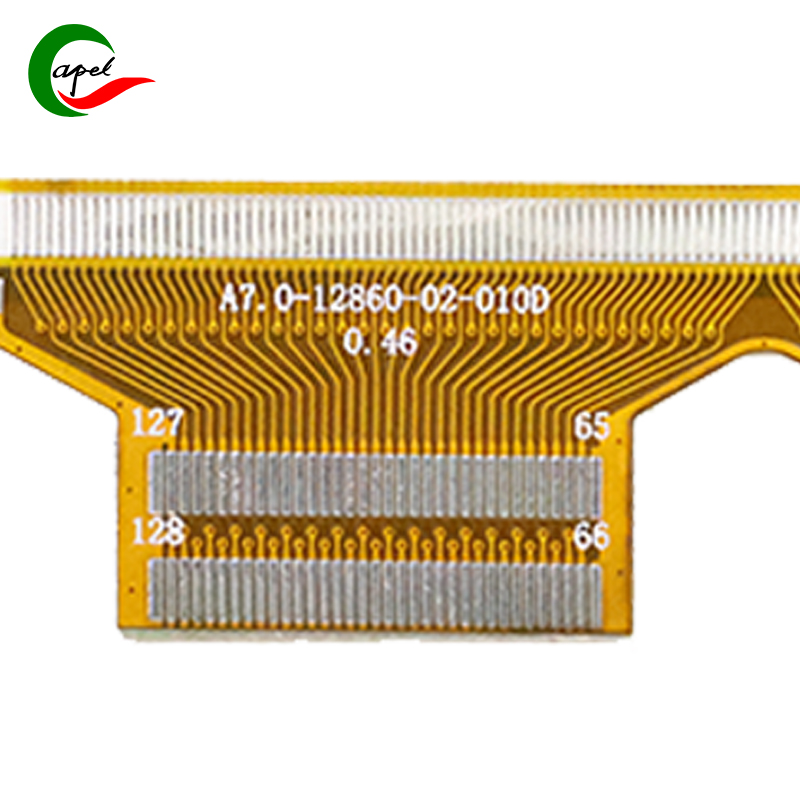
Featuring a 3-layer flexible core structure, it perfectly balances the space adaptability and operational stability of medical devices: with an overall thickness of 0.24mm, the flexible substrate offers excellent bending resilience, flexibly fitting narrow cavities, curved structures, and connections between dynamic components (e.g., instrument operation ends, module interfaces) inside medical devices. It easily avoids wiring interference, adapting to the compact design needs of portable and minimally invasive medical equipment; with precise dimensions of 17.5*62mm, the slim and compact layout perfectly matches the installation requirements of small-to-medium medical modules without redundant space. Backed by refined manufacturing standards, the 0.1/0.1mm ultra-fine equal line width/spacing and 0.2mm micro drill size enable ultra-high-density integration of signal and power channels, stably carrying transmission of multiple data groups (e.g., ECG, SpO2 signals, control commands) in limited space and providing solid circuit support for high-precision operation of medical devices.
Key advantages directly address medical scenario pain points, demonstrating medical-grade robustness: ① Equipped with 10-15μm thick tin plating, it offers excellent soldering reliability, resistance to sterilization wipes (compatible with medical disinfectants like alcohol and hydrogen peroxide), and corrosion resistance. Low contact resistance ensures high-speed, distortion-free signal transmission, eliminating data analysis errors caused by poor contact. Meanwhile, the thick tin layer extends product service life, adapting to long-term high-frequency use of medical devices; ② The 3-layer scientific wiring layout rationally separates signal, power, and ground layers, enabling separate transmission of signals and power to strongly suppress electromagnetic interference (EMI). It avoids signal crosstalk among multiple devices in medical environments, guaranteeing the integrity and accuracy of physiological data transmission, and complying with the IEC 60601 EMC standard for medical devices; ③ The flexible substrate meets medical-grade biocompatibility requirements, with excellent wide-temperature resistance (-40℃ to 85℃), strong fatigue resistance, and vibration resistance. It can withstand frequent operation, sterilization cycles, and transportation jolts of medical devices, reducing after-sales failure risks and aligning with the core demand of "low maintenance and high stability" in medical scenarios.
Backed by Kaboer’s years of medical-grade PCB R&D experience and strict quality control system, this 3-layer flexible PCB strictly complies with ISO 10993 biocompatibility standards and is compatible with global medical regulatory requirements (FDA, CE, NMPA). It can be customized for circuit layout, interface form, and wiring routes based on the functional needs of medical devices (e.g., sensor interface type, signal transmission protocol). Paired with rapid prototyping and efficient SMT assembly services, it helps customers shorten R&D cycles and accelerate product certification and market launch. Whether for precise signal collection of portable diagnostic devices, stable control of minimally invasive instruments, or real-time data transmission of monitors, it serves as the "core signal hub" of medical devices with core advantages of "regulatory compliance, accurate signal transmission, and stable durability", helping enterprises reduce compliance risks and enhance product credibility and market competitiveness!