This article explores the prototyping and manufacturing process of medical flexible PCBs, highlighting successful case studies from the medical industry. Learn about the complex challenges and innovative solutions encountered by experienced flexible PCB engineers, and gain insight into the critical role of prototyping, material selection, and ISO 13485 compliance in delivering reliable electronic solutions for medical applications.
Introduction: Medical Flexible PCBs in the Healthcare Industry
Flexible printed circuit boards (PCBs) play a vital role in the medical industry, where demanding applications require advanced and reliable electronic solutions. As a flexible PCB engineer with over 15 years of experience in the medical flexible PCB manufacturing industry, I have encountered and solved many industry-specific challenges. In this article, we’ll take a deep dive into the prototyping and manufacturing process for medical flexible PCBs and present a successful case study that highlights how our team solved a specific challenge for a customer in the medical industry.
Prototyping Process: Design, Testing, and Customer Collaboration
The prototyping stage is crucial when developing medical flexible circuit boards as it allows the design to be thoroughly tested and refined before entering mass production. Our team utilizes advanced CAD and CAM software to first create detailed schematics and layouts of flexible PCB designs. This process requires close collaboration with the customer to ensure that the design meets the specific requirements of the medical application, such as size constraints, signal integrity, and biocompatibility.
Case Study: Addressing Size Limitations and Biocompatibility
Addressing Dimensional Constraints and Biocompatibility
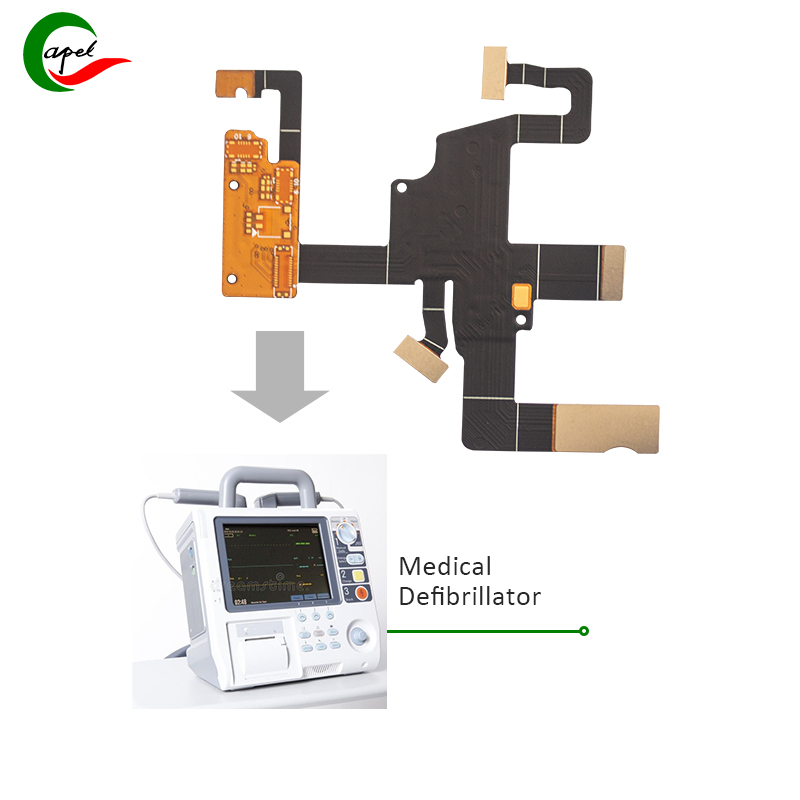
Our client, a leading medical device manufacturer, approached us with a challenging project requiring a miniaturized flexible PCB for implantable medical devices. The biggest concern for customers is the size constraints of the device, as it needs to be installed in a limited space while incorporating advanced sensor technology and wireless connectivity. Additionally, biocompatibility of the device is a critical requirement as it will be in direct contact with body fluids and tissues.
To address these challenges, our team began an extensive prototyping process, leveraging our expertise in miniaturization and biocompatible materials. The first phase involved conducting a thorough feasibility study to assess the technical feasibility of integrating the required components within the limited space. This requires working closely with the customer’s engineering team to understand functional requirements and performance expectations.
Using advanced 3D modeling and simulation tools, we iteratively optimized the flexible PCB layout to accommodate components while ensuring electrical integrity and signal isolation. In addition, we use specialized biocompatible materials, such as medical-grade adhesives and coatings, to mitigate the risk of tissue irritation and corrosion within implantable devices.
Medical Flexible PCB Manufacturing Process: Precision and Compliance
Once the prototyping phase has produced a successful design, the manufacturing process begins with precision and attention to detail. For medical flexible PCBs, the selection of materials and manufacturing techniques is critical to ensuring reliability, stability, and compliance with industry regulations such as ISO 13485 for medical devices.
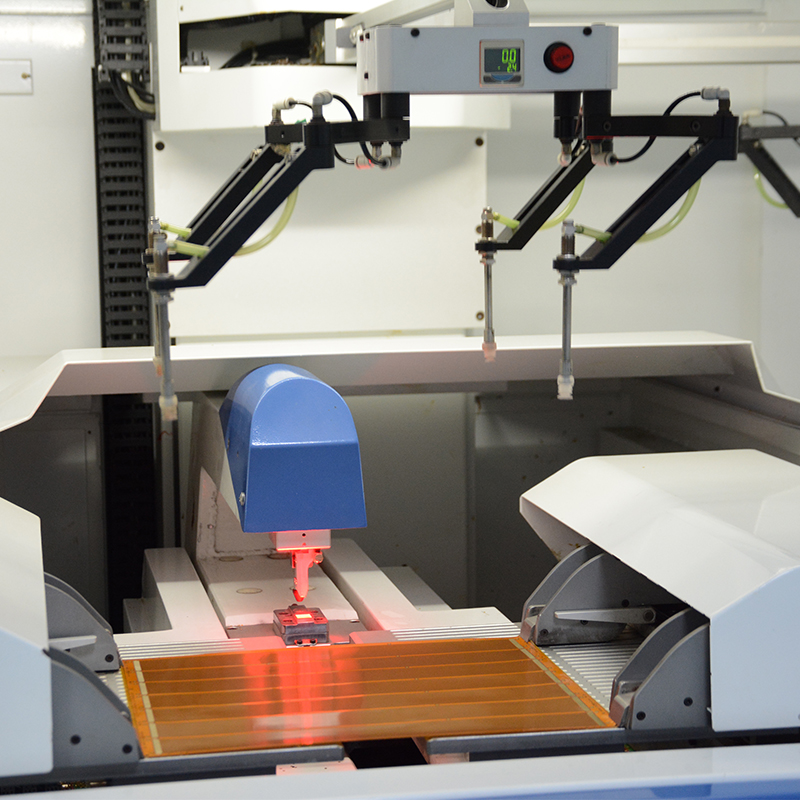
Our state-of-the-art manufacturing facility is equipped with state-of-the-art equipment specifically tailored for the production of medical flexible PCBs. This includes precision laser cutting systems for complex flex circuit patterns, controlled environment lamination processes that ensure the uniformity and integrity of multi-layer flex PCBs, and stringent quality control measures at every stage of production.
Case study: ISO 13485 compliance and material selection
ISO 13485 Compliance and Material Selection For an implantable medical device project, the client emphasized the importance of adhering to strict regulatory standards, specifically ISO 13485, to ensure the quality and safety of manufactured flexible PCBs. Our team works closely with customers to define the standards for material selection, process validation and documentation required for ISO 13485 certification.
To address this challenge, we conducted an in-depth analysis of compliant materials suitable for implantable medical devices, taking into account factors such as biocompatibility, chemical resistance, and reliability in long-term implant scenarios. This involves sourcing specialty substrates and adhesives that meet customer-specific requirements while complying with ISO 13485 standards.
In addition, our manufacturing processes are customized to incorporate rigorous quality control checkpoints such as automated optical inspection (AOI) and electrical testing to ensure that each flexible PCB meets required regulatory and performance standards. Close collaboration with customer quality assurance teams further facilitates the verification and documentation required for ISO 13485 compliance.
Medical Flexible PCB Prototyping and Manufacturing Process
Conclusion: Advancing Medical Flexible PCB Solutions
The successful completion of the miniaturized implantable medical device project highlights the critical role of prototyping and manufacturing excellence in solving industry-specific challenges in the medical flexible PCB space. As a flexible PCB engineer with extensive experience, I firmly believe that a combination of technical expertise, collaborative customer engagement, and compliance with industry standards are critical to delivering reliable and innovative solutions in the medical industry.
In conclusion, as our successful case study demonstrates, the prototyping and manufacturing process of medical flexible PCBs requires a keen understanding of the unique challenges of the medical field. The relentless pursuit of excellence in design, material selection and manufacturing practices is critical to ensuring the reliability and performance of flexible PCBs for critical medical applications.
By sharing this case study and insights into the prototyping and manufacturing process, our goal is to inspire further innovation and collaboration within the medical flexible PCB industry, driving the advancement of electronic solutions that can help improve healthcare outcomes.
As an experienced professional in the field of medical flexible PCBs, I am committed to continuing to solve industry-specific challenges and contribute to the development of electronic solutions that enhance patient care and medical technology.
Post time: Feb-28-2024
Back